Reverse Osmosis (RO) is a water purification technique that uses a semi-permeable membrane to get rid off ions, molecules and larger contaminants (particles) from fresh water.
What is Reverse Osmosis (RO)?
 |
| Image Courtesy: https://www.freshwatersystems.com/blogs/blog/reverse-osmosis-faqs |
Osmosis is a naturally occurring phenomenon and one of the foremost vital processes in nature. This is a method where less concentrated molecules (saline solution) will tend to migrate to more concentrated solution. The objective is an equalized solution.
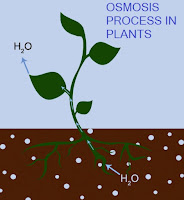
The examples of Osmosis are 'Cholera', 'plant roots drawing (absorb) water from the soil' and 'our kidney absorb water from our blood'.
In the below diagram we showed how osmosis works. A solution that is less concentrated will have a natural tendency to migrate to a solution with a higher concentration. For example, if a container filled with water with a low salt concentration and another container filled with water with high salt concentration and that they were separated by a semi-permeable membrane, then the water with the lower salt concentration would begin to migrate towards the water container with the higher salt concentration, until concentration becomes equal on both sides.
Semi-permeable membrane:
A semi-permeable membrane is a synthetic or biological membrane that will permit some atoms or molecules to pass but not others. A simple example for semi-permeable membrane is a screen door. It permits air molecules to go through but not pests or something larger than the holes within the screen door. Another example is blood brain barrier, it allows what ever the brain needs and what ever harmful is kept out. The membranes surrounding the organelles.
Reverse Osmosis:
Reverse osmosis is simply the opposite of osmosis method. Osmosis happens naturally without ant energy required. To reverse the method of osmosis we need to use some energy to the more saline solution. A Reverse Osmosis membrane is a semi-permeable membrane that acts like a filter having extremely tiny pores that enables the passage of water molecules however not the majority of dissolved microscopic contaminants like salts, organic, microorganism and pyrogens. However, we need to "push" the water through the reverse osmosis membrane (or semi-permeable membrane) by applying pressure that is greater than the naturally occurring osmotic pressure in-order to de-salinate (de-mineralize or de-ionize) water in the method, allowing pure water through while holding back majority of contaminants.
In the below diagram we showed the method of Reverse Osmosis. When pressure is applied to the concentrated solution the water molecules are forced through the semi-permeable membrane and the contaminants are not allowed through.
Semi-permeable membrane:
A semi-permeable membrane is a synthetic or biological membrane that will permit some atoms or molecules to pass but not others. A simple example for semi-permeable membrane is a screen door. It permits air molecules to go through but not pests or something larger than the holes within the screen door. Another example is blood brain barrier, it allows what ever the brain needs and what ever harmful is kept out. The membranes surrounding the organelles.
Reverse Osmosis:
Reverse osmosis is simply the opposite of osmosis method. Osmosis happens naturally without ant energy required. To reverse the method of osmosis we need to use some energy to the more saline solution. A Reverse Osmosis membrane is a semi-permeable membrane that acts like a filter having extremely tiny pores that enables the passage of water molecules however not the majority of dissolved microscopic contaminants like salts, organic, microorganism and pyrogens. However, we need to "push" the water through the reverse osmosis membrane (or semi-permeable membrane) by applying pressure that is greater than the naturally occurring osmotic pressure in-order to de-salinate (de-mineralize or de-ionize) water in the method, allowing pure water through while holding back majority of contaminants.
In the below diagram we showed the method of Reverse Osmosis. When pressure is applied to the concentrated solution the water molecules are forced through the semi-permeable membrane and the contaminants are not allowed through.